The Climate Basics – Matter, Energy, Atmosphere, and Oceans
(Reading #1 for my course on Climate Change - Alan Holyoak, PhD)
Note: This is the first reading in this series. This set of 7 readings is designed to help college general education students gain the the foundational background they need to understand the contents of the book "The Climate Crisis" by Archer and Rahmstorf, which I use as a course textbook.
Learning Objectives
By the end of this
topic (reading and class discussion) you should be able to understand and
explain:
- The difference between weather and climate
- The Law of Conservation of Matter
- The Laws of Thermodynamics
- The physical structure of the atmosphere
- The physical structure of the ocean
Introduction
In this reading you will review
some basic scientific principles that will help you start to build a foundation
of understanding of global climate and climate change. These include the Law of Conservation of
Matter, the Laws of Thermodynamics, and an introduction to the history, composition,
and structure of Earth’s atmosphere and ocean.
Physical Laws of Matter and Energy
The Law of Conservation of
Matter
The law of conservation of matter states
that matter cannot be created or destroyed, and that any changes to matter affect
only the form or chemical condition of the matter involved. This means that even if a substance undergoes
any physical change (vapor, liquid, solid) or chemical change, the resulting total
amount of matter you end up with is the same amount you started with.
The Laws of Thermodynamics
The laws of
thermodynamics describe the nature of energy.
The first law of thermodynamics
is also sometimes referred to as the law of conservation of energy. It states that energy cannot be created or
destroyed, and there is no increase or decrease in the total amount of energy in
a closed system. A closed system has no
energy entering or leaving it. This law
also states that heat will always move from a substance or location with higher
temperature to a substance or location with a lower temperature. This means that when you go outside on a cold
day you may feel like cold is seeping into your skin, but what is really
happening is that heat is seeping out of your body!
The second law of thermodynamics states the total amount of energy
available to do work decreases whenever energy is transformed from one form to
another. Entropy is unavoidably generated during any transformation of
energy and is released as waste heat. An
example of the second law is when your body converts food that you digest from
one chemical form into another one that you can use for energy. This conversion process is called respiration. Entropy that accumulates during respiration is
released as body heat. Another example
is when you burn gasoline in a car’s combustion engine. Some of the energy released is used to make
the car go, but entropy generated during that process is released as heat.
Introduction to Earth’s Atmosphere and Ocean
The Early Atmosphere and
Ocean
When the Earth formed 4.5 billion
years ago its atmosphere was significantly different than it is today and there
were no oceans – it was too hot for liquid water to exist. Gases that made up Earth’s first atmosphere
are listed in Table 1. The most abundant
gases were hydrogen and helium. Within 100
million years the atmosphere had changed significantly. A significant amount of hydrogen escaped into
space, and components of the primary atmosphere underwent chemical reactions to
make new additions to the atmosphere including water vapor and carbon dioxide,
but there was still no free oxygen!
Table 1. Components
of Earth’s ancient atmosphere (Table courtesy of Dr. Larry Hipps, Utah State
University).
Primary atmosphere
|
Atmosphere after
~100 million years
|
Hydrogen
|
Water vapor
|
Helium
|
Carbon Monoxide
|
Methane
|
Carbon Dioxide
|
Ammonia
|
Ammonia
|
Water vapor
|
Nitrogen
|
Sulfur Dioxide
|
|
Methane
|
Earth’s early atmospheric
temperature and pressure were also much different than what we experience
today. We currently have one atmosphere
of pressure at sea level, and an average global surface temperature of 15oC (59oF). Earth’s original atmosphere had a temperature
of 130-300oC (266-570oF) and an air pressure of about 256
atm. If you wanted to experience 256
atmospheres today you would have to descend to a depth of 2.6 km (1.6 miles)!
By about 4 billion years ago the
Earth cooled enough that the water vapor could condense and fall as rain. That initial downpour probably lasted for
thousands of years and filled the oceans.
Life appeared around 3.5 billion
years ago. This and other significant
events in Earth’s history are shown in Fig. 1.
The earliest life included only bacteria. These single-celled microbes included a group
called cyanobacteria. Cyanobacteria are important because
they did something new – photosynthesis.
Just in case you were struggling to
remember what photosynthesis is all
about, it is the process where an organism uses sunlight, water, and carbon
dioxide to make the energy-rich molecule glucose. Oxygen is the waste product of this
process. Twelve molecules of oxygen are
released for every molecule of glucose that is made: 6H2O + 6CO2
= C6H12O6 + 12O.
The concentration of free oxygen in
the early atmospheric remained very low, only a few parts per million, through
about 2.5 billion years ago even though cyanobacteria had been pumping out
oxygen for around a billion years. The
reason for the low levels of free oxygen is that most of the oxygen released
until this time was quickly bound up in chemical reactions in the air and
water. It is possible, however, that
there were “oxygen oases” in the sunlit surface waters of the ocean where
cyanobacteria lived, but oxygen diffusing out of the oases reacted almost
immediately with other molecules. The
atmosphere and all other areas and depths of the ocean therefore remained
virtually anoxic (oxygen free)(see
Fig. 2).
Figure 1. Significant
events in Earth’s atmospheric history (Figure courtesy of Dr. Hipps, Utah State
University).
Eventually the supply of molecules
that reacted with oxygen as soon as it was produced became saturated with
oxygen, so starting around 2.5 billion years ago oxygen concentrations in the
atmosphere and in oceanic surface waters started to increase. This is called “The Great Oxygenation Event.” This event sounds good to us since we need
oxygen to live, but at the time just about everything alive could not survive
in the presence of oxygen. Oxygen is a
highly reactive, poisonous gas, and the only reason it doesn’t kill us is that
oxygen is carefully chaperoned while it is in our bodies from the time it
enters our blood stream until it gets where it needs to go in our cells
(carried by hemoglobin in red blood cells, etc.). Early microbial species, however, lacked these
protective adaptations, and oxygen could kill them.
Gradually increasing concentrations
of oxygen in the atmosphere caused many species to go extinct because it disrupted
biological processes. Species that could
not find refuge from oxygen or adapt to withstand exposure to died off. As strange as it may sound, the accumulation
of oxygen in the ocean and atmosphere caused Earth’s first mass extinction event.
Figure 2. High
and low ranges of oxygen accumulation in the atmosphere (top), ocean surface
waters and shallow seas (middle), and deep ocean (bottom). (Figure modified from Holland, 2006.)
As the amount of oxygen increased
in the atmosphere, the concentration of carbon-containing molecules such as
carbon dioxide (CO2), carbon monoxide (CO), and methane (CH4)
decreased. Oxygen made up 2-5% of the
atmosphere by about 1.8 billion years ago, and surface waters of the ocean were
also oxygenated to comparable levels, but the deep sea remained anoxic. Then around 1.5 billion years ago a new form
of life appeared – eukaryotic organisms.
These organisms had a nucleus, some were photosynthetic, some were not,
but all of them needed oxygen to live. Also about that same time the oceans became as
salty as they are today. That salinity
and the salts that make it that way have not varied significantly since that
time, because as more salt is added to the ocean by the world’s rivers, salt is
removed from the ocean by chemical processes and sedimentation.
From 1.8 to about 0.8 billion years
ago there was no measurable change in the amount of oxygen in the atmosphere or
the oceans. This is called “The Boring Billion” years. One thing that did happen during this time is
that the deep sea became oxygenated – that’s probably where all the extra free
oxygen went. Then, starting around 850
million years ago oxygen levels began to rise dramatically until 540 million
years ago when it made up 10-20% of the atmosphere. This is when land plants proliferated and
added even more oxygen to the atmosphere.
Earth also experienced alternating glaciation and global hothouse
conditions. Toward the end of this time
period massive numbers and kinds of animal fossils appeared. This is called the Cambrian Explosion.
Lastly, there was a major spike in
the amount of oxygen in the atmosphere around 350 million years ago. At that time oxygen made up as much as 35% of
the atmosphere. This spike was probably produced
by the growth of Earth’s first massive forests between 400-300 million years
ago. Just in case you are curious, at
that time there were gigantic insects, such as dragonflies with wingspans of up
to 2.5 meters long! Soon after that
spike atmospheric oxygen levels dropped to 21%, its current concentration in
our modern atmosphere. The global
processes that maintain a constant 21% oxygen concentration are not yet well
understood.
The Modern Atmosphere
Earth’s modern atmosphere is the
result of geological, chemical, and biological processes that are too complex
to explain fully here – we will review and discuss aspects of this later. The composition of our modern troposphere
(the lowest layer of the atmosphere) is shown in Table 2. The major gases are nitrogen and oxygen that together
make up 99% of the troposphere. There are
several other gases present in small concentrations that have significant effects
on climate because they are greenhouse
gases – gases able to trap and hold energy as heat. Some of these greenhouse gases are water
vapor, carbon dioxide, methane, and chlorofluorocarbons (CFCs). CFCs are human-produced compounds used for
propellants, cleaning electronic components, and refrigeration and air
conditioning. The production of CFCs was
banned in the late 1980s they break down stratospheric ozone that protects us
from dangerous UV radiation. We will
examine the characteristics and effects of greenhouse gases in another reading
later on.
Earth’s atmosphere includes four
main layers. These are the troposphere,
stratosphere, mesosphere, and thermosphere.
The atmospheric layers are identified by the temperature profile shown
in Fig. 3.
The troposphere is lowest layer and extends to an altitude of 6-20 km
(3-12 mi). The troposphere is thickest
in the tropics and thinnest near the poles.
Eighty percent of the mass of the atmosphere and 99% of all water vapor
is found here. Virtually all weather
events take place in the troposphere.
The troposphere is warmest at the Earth’s surface where there is a
global average temperature of about 15oC (59oF), and
temperatures drop to about -45oC (-49oF) at the top of
the layer. This temperature difference exists
because there is a higher concentration of greenhouse gases near the planet
surface than there are near the top of the troposphere.
Heat from the sun heats the
troposphere, and friction between the lower troposphere and the earth’s surface
together with the Coriolis Effect produce
prevailing surface winds. The formation
of these surface winds will be discussed in a later reading. The atmospheric layer that lies on top of the
troposphere is the stratosphere.
Table 2.
Atmospheric components of the modern troposphere (Data courtesy of Dr. Hipps,
Utah State University).
Major Gases
|
Percentage of the
atmosphere
|
Nitrogen
|
~78%
|
Oxygen
|
~21%
|
Argon
|
<1%
|
Important Trace Greenhouse
Gases
|
Percentage of the
atmosphere
|
Water vapor
|
0.1-7%
|
Carbon Dioxide
|
~390 ppm (parts per million)
|
Methane
|
~1.7 ppm
|
N2O
|
~320 ppb (parts per billion)
|
CFC 11
|
~250 ppt (parts per trillion)
|
CFC 12
|
~540 ppt
|
The stratosphere extends to an altitude of about 50 km (30 mi). It contains most of the remaining 20% of the
mass of the atmosphere and it is extremely dry. Unlike the troposphere,
temperatures increase in the stratosphere with increasing altitude. The bottom of the stratosphere is coldest at
-45oC (-49oF), and the top is warmest at about -3oC
(27oF). This temperature
difference exists because ozone is produced
naturally at higher altitudes where oxygen (O2) is split apart by UV
radiation and individual oxygen molecules bond with molecules of atmospheric
oxygen (O2) to form ozone (O3). Ozone is extremely efficient at absorbing
ultraviolet radiation and some other wavelengths of light, thus warming the
stratosphere, but ozone allows many other wavelengths of light to pass through
and these wavelengths of light warm the troposphere as well as the Earth and
ocean surfaces.
The mesosphere, the layer above the stratosphere, extends from 50km (30
mi) to 85 km (53 mi) in altitude. There
are fewer gas molecules here than in the lower layers of the atmosphere, and the
concentration of molecules becomes progressively smaller as altitude
increases. Because of the small number
of molecules in the mesosphere, these molecules are constantly bombarded by the
complete spectrum of solar radiation. As
a result they are said to be in an excited state, i.e., they are constantly
absorbing and releasing energy.
Temperatures in the mesosphere range from -3oC (27oF)
at the lowest altitude to -93oC (-135oF) at the top. There are still enough molecules in the
mesosphere, though, that this is where meteorites become visible as “shooting
stars” as they burn up due to friction with the atmosphere.
Figure 3. Temperature
profile and layers of Earth’s atmosphere. (Figure from Wikimedia Commons.)
The outermost layer of the
atmosphere is the thermosphere. It extends to an altitude of about 600 km
(372 mi). There are very few molecules there. Because there are fewer molecules at
increasing altitudes there is less and less matter to absorb or block solar
radiation so temperatures climb from -93oC (-135oF) at
the bottom of the thermosphere to over 1700oC (3090oF) at
the top. The aurora borealis forms near
the base of the thermosphere, and the thermosphere is the layer of the atmosphere
where the shuttle flies and most satellites are in orbit.
The Earth’s Oceans
The Early Ocean
The size, shape, and location of
oceans change constantly but very slowly.
This is because the forces of plate tectonics move continents as new
oceanic crust is produced in some places and old oceanic crust is subducted (forced down) into the mantle
in other places. The resulting movement
of continental plates sometimes causes continents to be largely isolated from
each other as they are today, and at other times to be pushed together to form supercontinents. Supercontinents have existed at least five
times during Earth’s history. Their
names and the times they were formed are listed in Table 3. The most recent supercontinent was Pangea. It formed about 250 million years ago, about
the same time that dinosaurs appeared, and broke up between 200 and 65 million
years ago.
When a supercontinent exists the
rest of the Earth is covered by one massive ocean. When continents are mostly isolated, like
they are today, there are several smaller oceans. The locations and sizes of continents affect
the size, strength, and direction of surface and deep oceanic currents, and
these currents affect climate. We will
discuss the formation and effects surface and deep-water oceanic currents in a
later reading.
Table 3. Names and ages of some of Earth’s supercontinents.
Supercontinent name
|
Age formed
|
Vaalbara
|
3.6 billion years ago
|
Kenorland
|
2.7 billion years ago
|
Columbia or Nuna
|
1.8 billion years ago
|
Rodinia
|
1.0 billion years ago
|
Pangea
|
250 million years ago
|
Structure of the Ocean
Like the
atmosphere, the world’s oceans are separated into divisions based on the
conditions there. The bottom contour of
ocean basins is bounded on its edges by continental margins that include a continental shelf and a continental slope. The abyssal
plain is at the bottom of the continental slope. Mid-oceanic
ridges exist someplace in the middle of abyssal plains, as do oceanic trenches. New oceanic crust is formed along oceanic
ridges, and old crust material is subducted at trenches along plate margins (see Fig. 4).
Figure 4. Ages of
oceanic plate material and locations of mid-oceanic ridges and trenches. The youngest oceanic plate material is shown
in red and the oldest in blue. Examples
of ridges and trenches are indicated. (Image courtesy of NOAA.)
The
divisions of the ocean are shown in Fig. 5, and Fig. 6 may give you a better
handle of the relative scale of depth of the ocean and other water masses. The intertidal
zone is the thin margin of the ocean where seafloor is covered when tides
are high and are uncovered when tides are low.
All other parts of the ocean are referred to as the pelagic zone. The pelagic
zone is divided into two main subdivisions.
The portion of over continental slopes is called the neritic zone. The rest is called the oceanic zone. The pelagic
zone is also divided into horizontal layers by depth and other physical
conditions such as light penetration and temperature.
The surface
layer of the pelagic zone is called the epipelagic
zone. This layer usually extends to a depth of
several hundred meters. In most areas,
only about 1% of the sunlight that strikes the surface penetrates to the bottom
of the epipelagic zone. The epipelagic
zone is extremely important to Earth’s ecology because the majority of our planet’s
photosynthesis happens there, and this is where most marine life exists.
The mesopelagic zone is next deepest layer,
and extends from the bottom of the epipelagic zone to a depth where water
temperatures cool to ~10oC.
This zone is in perpetual twilight.
Most of the animals here have bioluminescence. This is an important layer because many
animals that live there migrate vertically into the epipelagic zone each
evening to feed on plankton and then migrate back to the mesopelagic zone each
morning. This vertical migration matters
because the migrating animals ingest huge amounts of carbon at the surface and
move that carbon to deeper waters.
The bathypelagic zone extends from the 10oC
temperature mark to the 4oC temperature depth. This is a zone of perpetual darkness. No light penetrates to this depth, and
animals there survive by eating each other and material that drifts down from
above.
Figure 5.
Divisions of the oceans. The 10oC
and 4oC lines are based on data from tropical waters in the Atlantic
Ocean. Actual depths of water at these
temperatures at a given location depend on latitude, season, and weather
conditions. The depth of the photic zone also varies with location, season, and
water conditions. (Image courtesy of
NOAA).
The abyssalpelagic
zone extends from the 4oC depth to the abyssal plain, which in
most places averages a depth of about 6,000 meters (3.7 miles). Animals that live there tend to stay close to
the bottom where they can pick food off of the bottom as well as particles that
drift down from above.
The hadalpelagic zone exists only where
there are oceanic trenches, and can extend to depths greater than 10,000 meters
(6 miles). Little is known about life at
these depths, but fishes and invertebrates are known to exist in trenches.
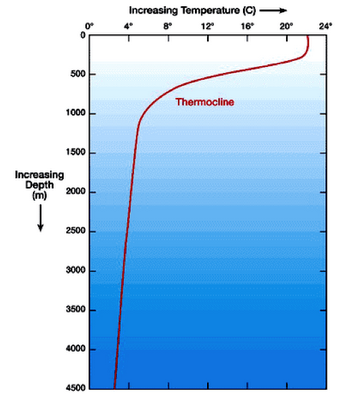
The
vertical profile of ocean water from the surface downward to deeper water also
includes a thermocline (Fig.
7). A thermocline is the depth of water
where there is rapid temperature change with increasing depth. The thermocline is particularly important when
measuring and considering the movement of heat and nutrients throughout the
ocean, because water above the thermocline is warmer and less dense than the
water below the thermocline, and water of different densities do not easily
mix.
Oceanographers
have discovered complex sets of surface and deep-water currents in the
oceans. These currents carry water,
plankton, dissolved gases, heat, and other things that affect the global
climate. We will investigate the
formation and effects of these currents later in the course.
Figure 6. Depths
of the ocean and some major lakes. Vertical depth scales are accurate,
horizontal scales are not. (Courtesy of
aquaticsports.com)
Figure 7. The
thermocline exists where when water undergoes rapid temperature change in its
vertical profile. (Image courtesy of
marinebio.org)
Review Questions
1.
What
does the first law of thermodynamics have to do with climate?
2.
Why
does it matter that we know that the atmosphere has changed in the past?
3.
Why are
the trace gases in the modern atmosphere so important?
4.
Why
does tectonic movement of the continents matter to global climate?
5.
Why
does it matter that you have a basic understanding of the structure of the ocean?
Source material
Hipps, LE. 2010. Personal
communication and materials produced and provided by Dr. Hipps. Professor of
Atmospheric Science, Dept of Plants, Soils, and Climate. Utah State University.
Holland, HD. 2006. The
oxygenation of the atmosphere and oceans. Philosophical Transactions of The
Royal Society Ser. B. 361 (1470): 903-915.
Nybakken,
JW, and MD Bertness. 2005.
Marine Biology: An Ecological Approach. 6th Edition. Pearson
Benjamin Cummings Publishers, N. Y. 578






No comments:
Post a Comment